
This web site, https://www.vcru.wisc.edu/sdata, contains data from the laboratory of Philipp W. Simon, USDA-ARS Vegetable Crops Research Unit [Click here for our web page]
 |
USDA ARS VCRU Data Server This web site, https://www.vcru.wisc.edu/sdata, contains data from the laboratory of Philipp W. Simon, USDA-ARS Vegetable Crops Research Unit [Click here for our web page] |
BIOMETRICAL STUDIES AND QUANTITATIVE TRAIT LOCI ASSOCIATED WITH MAJOR PRODUCTS OF
THE CAROTENOID PATHWAY OF CARROT (DAUCUS CAROTA L.)
by Carlos Antonio Fernandes Santos
Ph.D. Thesis, University of Wisconsin-Madison, 2001
Chapter 3: Construction of AFLP based linkage maps in F2 populations of carrot derived from
crosses between wild white (Daucus carota L. var. carota) x cultivated orange (Daucus
carota L. ssp. sativus) and cultivated orange x cultivated dark orange
This population was referenced in Genetics and Molecular Biology, 25, 2, 195-201 (2002)
Carlos A.F. Santos and Philipp W. Simon
Some AFLP amplicons are highly conserved DNA sequences mapping to the
same linkage groups in two F2 populations of carrot
Abstract Amplified fragment length polymorphism (AFLP) is a fast and reliable tool to generate a large number of DNA markers. In two unrelated F2 populations of carrot ( Daucus carotaL.), Brasilia x HCM and B493 x QAL (wild carrot), it was hypothesized that DNA 1) digested with the same restriction endonuclease enzymes and amplified with the same primer combination and 2) sharing the same position in polyacrylamide gels should be conserved sequences. To test this hypothesis AFLP fragments from polyacrylamide gels were eluted, reamplified, separated in agarose gels, purified, cloned and sequenced. Among thirty-one paired fragments from each F2 population, twenty-six had identity greater than 91% and five presented identity of 24% to 44%. Among the twenty-six conserved AFLPs only one mapped to different linkage groups in the two populations while four of the five less-conserved bands mapped to different linkage groups. Of eight SCAR (sequence characterized amplified regions) primers tested, one conserved AFLP resulted in co-dominant markers in both populations. Screening among 14 carrot inbreds or cultivars with three AFLP-SCAR primers revealed clear and polymorphic PCR products, with similar molecular sizes on agarose gels. The development of co-dominant markers based on conserved AFLP fragments will be useful to detect seed mixtures among hybrids, to improve and to merge linkage maps and to study diversity and phylogenetic relationships.
This population was referenced in Mol Genet Genomics (2002) 268: 122–129
C.A.F. Santos, P.W. Simon
QTL analyses reveal clustered loci for accumulation of major provitamin A carotenes and lycopene in carrot roots
Abstract QTLs associated with products of the carotenoid pathway, including lycopene and the provitamin A carotenes α- and β-carotene, were investigated in two unrelated F2 carrot populations, derived from crosses between orange cultivated B493 and white wild QAL (Population 1), and orange cultivated Brasilia and darkorange cultivated HCM (Population 2). The mapping populations of 160 and 180 individuals, respectively, were analyzed with single-marker and interval-mapping statistical approaches, using coupling linkage maps for each parent. Single markers were selected for further analysis based on the Wilcoxon sum-rank non-parametric test. Interval mapping performed with Population 2 detected four, eight, three, one and five putative QTLs associated with accumulation of ζ-carotene, α-carotene, β-carotene, lycopene and phytoene, respectively. Among these, the major QTLs explained 13.0%, 10.2%, 13.0%, 7.2% and 10.2% of total phenotypic variation. In Population 1 single-marker analysis identified loci explaining up to 13.8%, 6.8%, 19.3%, 5.7%, and 17.5%, respectively, of total phenotypic variation for these same carotenoids. Overall analysis demonstrated clustering of these QTLs associated with the carotenoid pathway: the AFLP loci AACCAT178-Q and AAGCAG233-Q, on linkage group 5, explained 17.8%, 22.8% and 23.5% of total phenotypic variation for ζ-carotene, phytoene and β-carotene in Population 1. Two major clusters of QTLs, with LOD scores greater than 1.8, mapped tointervals no larger than 2 cM for ζ-carotene, β-carotene, α-carotene and lycopene on linkage group 3, and for ζ-carotene and phytoene on linkage group 9, and these explained 3.7% to 13.0% of variation for each carotenoid product. Thus, these results suggest that clustering of related pathway loci is favored during evolution, since closely linked "pathway mates" are not easily separated by recombination.
This population was referenced in J. Amer. Soc. Hort. Sci. 129(2): 211-217. 2004.
Carlos A. F. Santos, Philipp W. Simon
Merging Carrot Linkage Groups based on Conserved Dominant AFLP Markers in F2 Populations
Abstract Markers were placed on linkage groups, ordered, and merged for two unrelated F2 populations of carrot (Daucus carota L.). Included were 277 and 242 dominant AFLP markers and 10 and 8 co-dominant markers assigned to the nine linkage groups of Brasilia × HCM and B493 × QAL F2 populations, respectively. The merged linkage groups were based on two co-dominant markers and 28 conserved dominant AFLP markers shared by both populations. The average marker spacing was 4.8 to 5.5 cM in the four parental coupling phase maps. The average marker spacing in the six merged linkage groups was 3.75 cM with maximum gaps among linkage groups ranging from 8.0 to 19.8 cM. Gaps of a similar size were observed with the linkage coupling phase maps of the parents, indicating that linkage group integration did not double the bias which comes with repulsion phase mapping. Three out of nine linkage groups of carrot were not merged due the absence of common markers. The six merged linkage groups incorporated similar numbers of AFLP fragment from the four parents, further indicating no significant increase in bias expected with repulsion phase linkage. This is the first report to use shared conserved AFLPs to merge linkage maps. The genome coverage in this study is suitable to apply QTL analysis and to construct a cross-validated consensus map of carrot. This initial effort takes a large step toward an integrated map of carrot.
Details about individual markers are available by clicking on any of the markers on the map, or by going directly to the marker information page
Mapping populations were obtained by self-pollinating single F1 hybrid plants which originated from a cross between B493 × QAL (inbred line × wild carrot; cross 1) and Brasilia × HCM (open-pollinated Brazilan cultivar × high carotene population; cross 2). Leaf samples from 183 and 160 F2 plants, from cross 1 and 2 respectively, were harvested and prepared to DNA extraction.
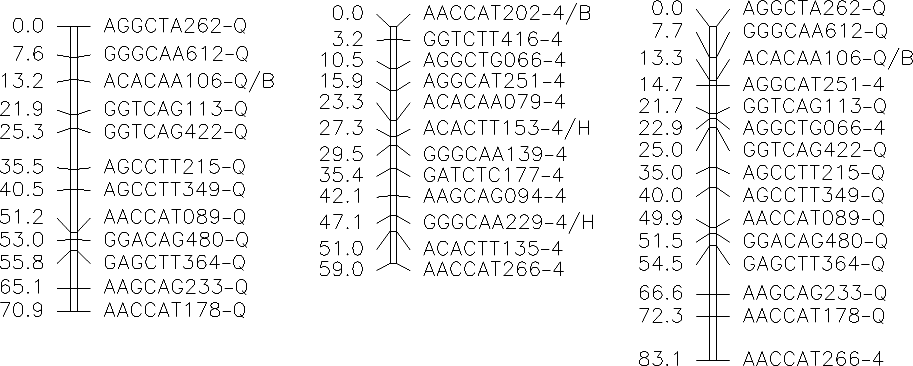
The following figures represent the linkage groups for the B493 × QAL F2 population of carrot. AFLP markers are named by (1) the code of the primer combination, followed by (2) the estimated molecular size in nucleotides and (3) a code indicating the parental origin of the fragment. AFLP fragments with – and / represent a conserved AFLP fragment between the two populations and a co-dominant AFLP, respectively.
| Group 1QC QAL-Coupling | Group 1BC B493-Coupling | Group 1CR Coupling+Repulsion | Links to Marker Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group 2QC QAL-Coupling | Group 2BC B493-Coupling | Group 2CR Coupling+Repulsion | Links to Marker Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
XXX | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group 3QC QAL-Coupling | Group 3BC B493-Coupling | Group 3CR Coupling+Repulsion | Links to Marker Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
XXX | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group 4QC QAL-Coupling | Group 4BC B493-Coupling | Group 4CR Coupling+Repulsion | Links to Marker Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
XXX | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group 5QC QAL-Coupling | Group 5BC B493-Coupling | Group 5CR Coupling+Repulsion | Links to Marker Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
XXX | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group 6QC QAL-Coupling | Group 6BC B493-Coupling | Group 6CR Coupling+Repulsion | Links to Marker Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
XXX | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group 7QC QAL-Coupling | Group 7BC B493-Coupling | Group 7CR Coupling+Repulsion | Links to Marker Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
XXX | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group 8QC QAL-Coupling | Group 8BC B493-Coupling | Group 8CR Coupling+Repulsion | Links to Marker Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
XXX | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group 9QC QAL-Coupling | Group 9BC B493-Coupling | Group 9CR Coupling+Repulsion | Links to Marker Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
XXX | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||